The chemistry of steel determines why and how it should be tempered

Steels ain’t steels to paraphrase an old advertisement.
At least they are not all the same. Steel is an alloy, an amalgam of materials – primarily iron and carbon. Carbon is the defining characteristic of steel types and steel is usually classified according to the amount of carbon it contains:
Low carbon steel
up to 0.15% carbon
Medium carbon steel
0.30-0.59% carbon
High carbon steel
0.60-0.90% carbon
Tool steels
0.70-1.50% carbon
The potential hardness of a steel is determined by its carbon content. Adding small amounts of other elements to the mix can produce enormous improvements in strength, toughness, and
resistance to rust and the response of the steel to heat treatment.
Steels referred to as alloy steels have additional elements such as chromium, nickel, vanadium,
cobalt, manganese, molybdenum, silicon, and tungsten included. Low-alloy steels, steels where the alloy content is less than five percent, have a higher tensile strength than mild steel. Small amounts of chromium or molybdenum added to carbon steel improve its strength at higher temperatures.
High-alloy steel has more than five percent of alloys in the mix, typically over 10 percent, which
gives them outstanding properties in various areas. Stainless steel for example contains high percentages of chromium and nickel.
Crystalline
Steel, like all metals, has a crystalline structure. The nature of the crystalline structure is determined by the various inclusions in the iron and the speed with which it cools. If it is allowed to cool slowly, the crystal structure will be fine with small crystals finely divided
throughout the material. This type of steel is generally quite malleable and ductile.
If the steel is cooled suddenly, however, the crystals formed are large and more susceptible to
shearing and cracking. This type of steel is brittle but quite hard. The crystal structure of steel is often referred to as its microstructure and it is this that is altered during the hardening and tempering process.
Carbon dissolves readily in iron. At a temperature of around 723° C the microstructure of steel begins to change. Carbon grains dissolve in the iron and mix within the iron crystal structure in a form known as a solid solution. Between 750° and 820° C, a point known as the “upper critical temperature” (UCT), steel loses its magnetism.
Austenite
This is also the region of temperature within which the carbon grains within the steel dissolve and the material takes on a structure known as austenite.
If steel is cooled rapidly from this point, the carbon is retained in the iron in the form of a super-saturated solution known as martensite. Martensite is harder and more brittle, but it is this which gives steel its hardness.
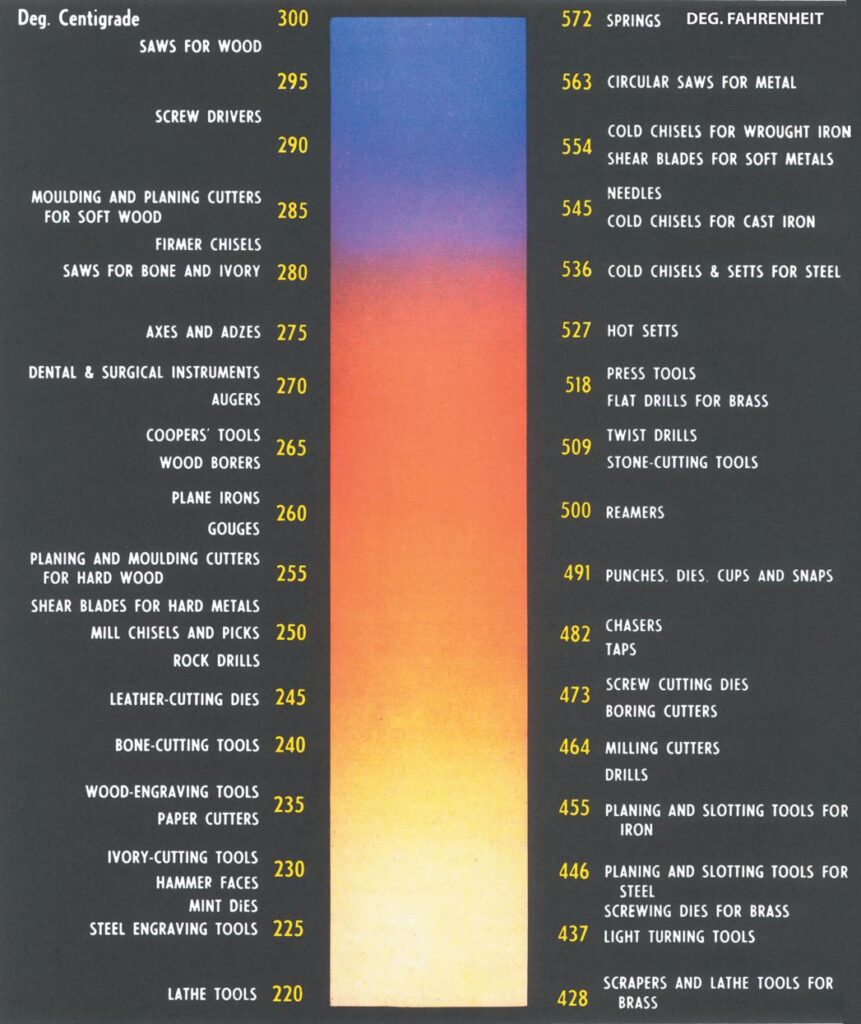
To be useful, though, the stresses within the lattice need to be relieved a little and this is what occurs during the tempering process, where the steel is heated again to around 200° to 300° C and
quenched. Temperature is critical, but steel has the advantage that as it heats up its oxides emit a range of colours that relate very closely to the temperature of the steel (see chart above).
The transition from red to orange corresponds to the critical limits in steel. Quenching can be done in water, brine, or oil. Water has the benefit of cooling the steel very rapidly but not necessarily as evenly as oil quenching, which cools the steel more slowly. There are also advantages in cooling steel from room temperature to below zero.
The cryogenic-like temperature will transform some vestigial austenite in the steel into martensite at these low temperatures.

